What are the reasons for the cause Lithium ion battery fire?
Why battery safety is important
Lithium-ion batteries were developed in the 1970s and first launched by Sony in 1991 for the company’s portable video recorder.
Today, everything you see is powered by batteries, from smartphones to electric cars and even the International Space Station.
In 2008, Tesla unveiled the Roadster, making it the first car company to market a battery-powered electric vehicle. By 2025, the global lithium-ion (Li-ion) battery market is expected to reach $100.4 billion, more than 50% of which will be used for the car market.
Why such a craze for lithium-ion?
Lithium-ion batteries are popular because of the power they can deliver at a given size and weight. A typical lithium-ion battery stores 150 watt-hours of electricity in 1 kilogram of battery, compared to a NiMH battery (100 watt-hours per kg) or a lead-acid battery (25 watt-hours per kg).To store the same amount of energy in a lead-acid battery that a 1-kilogram lithium-ion battery can handle, 6 kilograms are required.
However, lithium-ion batteries are extremely sensitive to high temperatures and are inherently flammable.These batteries tend to deteriorate much faster than normal due to heat. If a lithium-ion battery fails, it bursts into flames and can cause extensive damage. This calls for immediate action and guidelines on battery safety.
Recently, there have been some incidents of fires caused by lithium-ion batteries. On 8 January 2019, the spontaneous combustion of a lithium-ion battery caused a fire to break out on the COSCO Pacific, a ship in the Arabian Sea. In April last year, a 2MW battery exploded at an APS plant in Arizona, injuring four firefighters.
Hans-Otto Schjerven, chief of the Vestfold fire service, said rechargeable lithium batteries “can cause fires that are difficult to extinguish and the batteries give off fire that spreads quickly”. As the use of electric vehicles increases, these incidents will increase.
Before we analyze why lithium-ion batteries catch fire, we need to understand how they work.
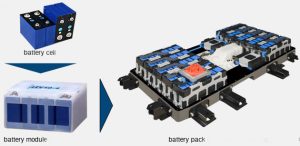
A lithium-ion battery pack consists of lithium-ion cells stacked in modules, temperature sensors, voltage gauges and an onboard computer (Battery Management System) to manage the individual cells. Like any other cell, the lithium-ion cell has a positive electrode (cathode), a negative electrode (anode) and a chemical called electrolyte in between. While the anode is usually made of graphite (carbon), different lithium materials are used for the cathode – Lithium Cobalt Oxide (LCO), Lithium Nickel Manganese Cobalt (or NMC), etc.
When the cell is charged, lithium ions move through the electrolyte from the cathode to the anode. The electrons also flow but take the longer path outside the circuit. The opposite movement takes place during discharge, resulting in the electron’s power going up to the application to which the cell is connected.
When all ions have returned to the cathode, the cell is fully discharged and must be recharged.
The lithium-ion cells are designed with battery safety measures such as:
A. Pressure-sensitive vent holes
Batteries are pressurized and therefore require a metal outer wall equipped with a pressure-sensitive vent hole. If the battery threatens to become very hot and explode due to overpressure (pressure build-up at 3,000 kPa), this vent allows the extra pressure to escape and prevents other cells in the battery from catching fire.
B. Separator serves as a fuse
Most lithium-ion cells use a separator made of a material known as polyolefin, which has good chemical stability, excellent mechanical properties and is affordable. It serves as a fuse when the cell heats up. Upon overheating, when the core reaches 130°C (266°F), the separator melts, stopping the transport of ions. This action immediately switches off the cell.
Had this feature not been fitted, it would have been possible for the heat in the failing cell to reach the thermal runaway threshold and ignite with flames.
C. Positive temperature coefficient (PTC)
This is a switch that prevents the battery from overheating by protecting it from current surges.
Lithium-ion cells, like all other types of chemistry, undergo self-discharge. Self-discharge means that the batteries lose their stored charge without connecting the electrodes or the external circuit. This happens due to chemical reactions inside the cell. Self-discharge of cells increases with age, cycles and high temperatures.
Increased self-discharge can cause the temperature to rise, which, if discharged uncontrolled, can lead to a thermal runaway, also known as ‘venting with flame’. A slight short circuit will not cause a thermal runway because the discharge energy is very low and little heat is generated.
However, if some damage to the cell causes impurities to enter the cell, a large electrical short circuit may occur and a significant current will flow between the positive and negative plates. The temperature rises suddenly and the energy stored in the battery is released within milliseconds. Battery packs consist of thousands of cells packed together.
During a thermal runaway, heat from a faulty cell can travel to the next cell, making it thermally unstable as well. This chain reaction can result in the entire pack being destroyed within seconds.
Now that we know why lithium-ion batteries catch fire, let's look at the reasons why this can happen:
A. Manufacturing defects
Manufacturing errors can cause metal particles (impurities) to enter the lithium-ion cell during the manufacturing process. Battery manufacturers should ensure strictly controlled clean rooms for battery manufacturing.
Another defect can be the thinning of the separators, which can be harmful during actual use. Cells should undergo strict quality control and be validated before being sold.
B. Design flaws
Car manufacturers want their cars to be as sleek and streamlined as possible while offering maximum range and performance. These requirements force battery pack manufacturers to come up with compact designs by cramming high-capacity cells into a smaller housing, compromising an otherwise well-built battery.
By compromising the design, the electrodes or the separator can be damaged. Both can lead to short circuits. Furthermore, the lack of a proper cooling system or venting can raise the temperature of the battery as the flammable electrolyte heats up.
If this is not controlled, it can lead to a chain reaction of faulty cells, causing the battery to heat up further and get out of control.
C. Abnormal or improper use
External factors, such as keeping the battery very close to a heat source or near a fire, may cause an explosion. Intentional or accidental penetration of the battery may cause short circuits and fire. Therefore, unauthorized dismantling of the battery pack in electric vehicles will result in voiding the warranty.
Users are advised to have batteries checked and repaired only by authorized service centers of the vehicle manufacturer. Even charging under high voltage or excessive discharge of the battery pack can damage it.
D. Problems with chargers
Using poorly insulated chargers may damage the battery. If the charger short circuits or generates heat near the battery, it may cause enough damage to cause a failure.
Although lithium-ion batteries have built-in safeguards to prevent overcharging, using unofficial chargers can damage the battery in the long run.
E. Low-quality components
Besides manufacturing defects, one of the main causes of battery failures is the use of low-quality parts. With increasing competition, battery prices are falling, causing battery manufacturers to cut corners. Skimping on low-quality electronics, such as the battery management system, increases the risk of battery failures.
The battery management system is essential for battery safety and performance. It protects the battery from operating outside its safe operating range. Since batteries are a high-value component of an electric vehicle or energy storage system, it is essential to invest in a smart battery management system that can immediately detect cell faults and prevent the battery from exploding.
How to ensuring battery safety
Battery pack manufacturers should take an uncompromising approach to battery safety. Lithium-ion batteries can be made safer by making them “smart”. By building an intelligence layer into batteries, we can not only diagnose but also predict abnormal usage or abnormal battery performance. This allows us to intervene in time, prevent damage to the system and ensure the safety of the battery.
If you want to know more about lithium-ion battery safety, write to us at service@liionakb.ru








Leave a reply